You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004680_01216
You are here: Home > Sequence: MGYG000004680_01216
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
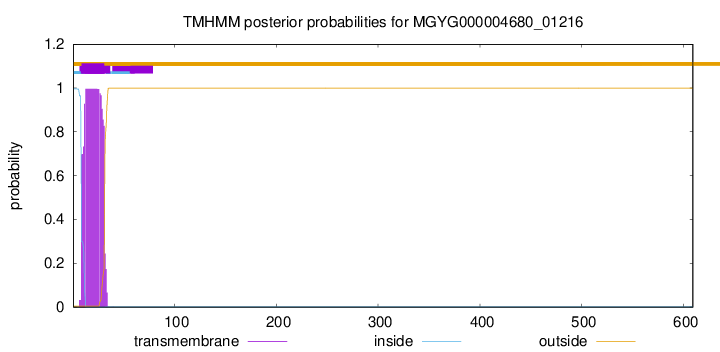
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; Lacrimispora; | |||||||||||
| CAZyme ID | MGYG000004680_01216 | |||||||||||
| CAZy Family | CBM50 | |||||||||||
| CAZyme Description | Mannosylglucosyl-3-phosphoglycerate phosphatase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 21485; End: 23314 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG0737 | UshA | 4.11e-74 | 11 | 600 | 1 | 510 | 2',3'-cyclic-nucleotide 2'-phosphodiesterase/5'- or 3'-nucleotidase, 5'-nucleotidase family [Nucleotide transport and metabolism, Defense mechanisms]. |
| PRK09419 | PRK09419 | 1.71e-66 | 35 | 609 | 659 | 1160 | multifunctional 2',3'-cyclic-nucleotide 2'-phosphodiesterase/3'-nucleotidase/5'-nucleotidase. |
| PRK09558 | ushA | 2.00e-66 | 7 | 602 | 1 | 546 | bifunctional UDP-sugar hydrolase/5'-nucleotidase periplasmic precursor; Reviewed |
| cd00845 | MPP_UshA_N_like | 2.60e-51 | 37 | 289 | 1 | 239 | Escherichia coli UshA-like family, N-terminal metallophosphatase domain. This family includes the bacterial enzyme UshA, and related enzymes including SoxB, CpdB, YhcR, and CD73. All members have a similar domain architecture which includes an N-terminal metallophosphatase domain and a C-terminal nucleotidase domain. The N-terminal metallophosphatase domain belongs to a large superfamily of distantly related metallophosphatases (MPPs) that includes: Mre11/SbcD-like exonucleases, Dbr1-like RNA lariat debranching enzymes, YfcE-like phosphodiesterases, purple acid phosphatases (PAPs), YbbF-like UDP-2,3-diacylglucosamine hydrolases, and acid sphingomyelinases (ASMases). MPPs are functionally diverse, but all share a conserved domain with an active site consisting of two metal ions (usually manganese, iron, or zinc) coordinated with octahedral geometry by a cage of histidine, aspartate, and asparagine residues. The conserved domain is a double beta-sheet sandwich with a di-metal active site made up of residues located at the C-terminal side of the sheets. This domain is thought to allow for productive metal coordination. |
| pfam02872 | 5_nucleotid_C | 4.52e-44 | 391 | 566 | 1 | 155 | 5'-nucleotidase, C-terminal domain. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADL06114.1 | 0.0 | 1 | 609 | 1 | 609 |
| QRV19771.1 | 0.0 | 1 | 609 | 1 | 609 |
| SET94084.1 | 0.0 | 1 | 609 | 1 | 609 |
| ASN98642.1 | 2.82e-306 | 24 | 609 | 29 | 614 |
| QRP42178.1 | 2.82e-306 | 24 | 609 | 29 | 614 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1USH_A | 2.02e-33 | 8 | 586 | 2 | 531 | 5'-NucleotidaseFrom E. Coli [Escherichia coli K-12],2USH_A 5'-Nucleotidase From E. Coli [Escherichia coli K-12],2USH_B 5'-Nucleotidase From E. Coli [Escherichia coli K-12] |
| 1HO5_A | 2.35e-32 | 37 | 586 | 9 | 506 | 5'-Nucleotidase(E. Coli) In Complex With Adenosine And Phosphate [Escherichia coli],1HO5_B 5'-Nucleotidase (E. Coli) In Complex With Adenosine And Phosphate [Escherichia coli],1HPU_A 5'-Nucleotidase (Closed Form), Complex With Ampcp [Escherichia coli],1HPU_B 5'-Nucleotidase (Closed Form), Complex With Ampcp [Escherichia coli],1HPU_C 5'-Nucleotidase (Closed Form), Complex With Ampcp [Escherichia coli],1HPU_D 5'-Nucleotidase (Closed Form), Complex With Ampcp [Escherichia coli] |
| 4WWL_A | 2.55e-32 | 37 | 586 | 9 | 506 | E.coli 5'-nucleotidase mutant I521C labeled with MTSL (intermediate form) [Escherichia coli K-12] |
| 1OID_A | 8.39e-32 | 37 | 586 | 9 | 506 | 5'-Nucleotidase(E. coli) with an Engineered Disulfide Bridge (S228C, P513C) [Escherichia coli],1OID_B 5'-Nucleotidase (E. coli) with an Engineered Disulfide Bridge (S228C, P513C) [Escherichia coli],1OIE_A 5'-Nucleotidase (E. coli) with an Engineered Disulfide Bridge (S228C, P513C) [Escherichia coli] |
| 1HP1_A | 9.63e-32 | 37 | 586 | 9 | 497 | 5'-Nucleotidase(Open Form) Complex With Atp [Escherichia coli] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| A9BJC1 | 1.79e-42 | 12 | 585 | 5 | 481 | Mannosylglucosyl-3-phosphoglycerate phosphatase OS=Petrotoga mobilis (strain DSM 10674 / SJ95) OX=403833 GN=mggB PE=1 SV=1 |
| O34313 | 1.54e-36 | 36 | 606 | 668 | 1174 | Trifunctional nucleotide phosphoesterase protein YfkN OS=Bacillus subtilis (strain 168) OX=224308 GN=yfkN PE=1 SV=1 |
| Q9KQ30 | 7.93e-36 | 33 | 585 | 32 | 533 | 5'-nucleotidase OS=Vibrio cholerae serotype O1 (strain ATCC 39315 / El Tor Inaba N16961) OX=243277 GN=nutA PE=3 SV=1 |
| P22848 | 9.75e-35 | 39 | 595 | 40 | 543 | 5'-nucleotidase OS=Vibrio parahaemolyticus serotype O3:K6 (strain RIMD 2210633) OX=223926 GN=nutA PE=3 SV=2 |
| P07024 | 1.11e-32 | 8 | 586 | 2 | 531 | Protein UshA OS=Escherichia coli (strain K12) OX=83333 GN=ushA PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
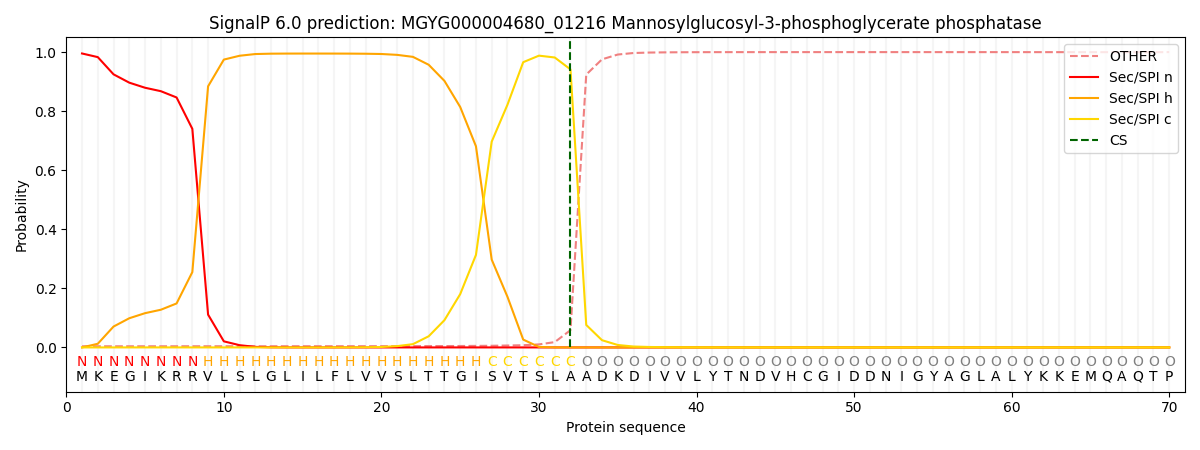
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.005342 | 0.992707 | 0.000994 | 0.000477 | 0.000239 | 0.000211 |