You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004854_01896
You are here: Home > Sequence: MGYG000004854_01896
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; Ruminococcus; | |||||||||||
| CAZyme ID | MGYG000004854_01896 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 111611; End: 114331 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 224 | 439 | 7.5e-31 | 0.8960396039603961 |
| CBM13 | 566 | 715 | 1.6e-20 | 0.7978723404255319 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3866 | PelB | 8.87e-29 | 173 | 514 | 30 | 340 | Pectate lyase [Carbohydrate transport and metabolism]. |
| pfam14200 | RicinB_lectin_2 | 1.88e-17 | 594 | 681 | 4 | 89 | Ricin-type beta-trefoil lectin domain-like. |
| smart00656 | Amb_all | 2.14e-15 | 244 | 439 | 17 | 187 | Amb_all domain. |
| cd14256 | Dockerin_I | 1.80e-13 | 848 | 904 | 1 | 57 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
| pfam00544 | Pec_lyase_C | 1.99e-09 | 235 | 435 | 26 | 209 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBL17651.1 | 0.0 | 30 | 904 | 31 | 893 |
| CDM70399.1 | 7.63e-120 | 4 | 694 | 3 | 694 |
| AUO18238.1 | 1.09e-118 | 26 | 694 | 25 | 705 |
| CBL17517.1 | 1.57e-98 | 501 | 903 | 450 | 849 |
| CBL16296.1 | 3.11e-91 | 548 | 870 | 613 | 945 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3VMV_A | 1.32e-07 | 175 | 448 | 2 | 256 | Crystalstructure of pectate lyase Bsp165PelA from Bacillus sp. N165 [Bacillus sp. N16-5],3VMW_A Crystal structure of pectate lyase Bsp165PelA from Bacillus sp. N165 in complex with trigalacturonate [Bacillus sp. N16-5] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O34819 | 5.56e-16 | 175 | 518 | 34 | 339 | Pectin lyase OS=Bacillus subtilis (strain 168) OX=224308 GN=pelB PE=3 SV=1 |
| P94449 | 5.56e-16 | 175 | 518 | 34 | 339 | Pectin lyase OS=Bacillus subtilis OX=1423 GN=pelB PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
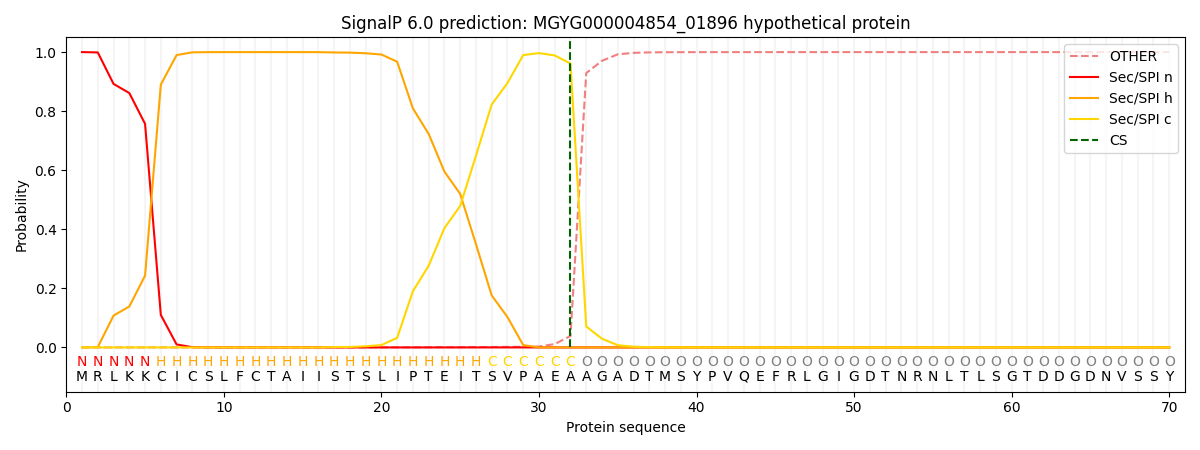
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000244 | 0.999087 | 0.000201 | 0.000173 | 0.000151 | 0.000133 |
