You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004876_00931
You are here: Home > Sequence: MGYG000004876_00931
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; | |||||||||||
| CAZyme ID | MGYG000004876_00931 | |||||||||||
| CAZy Family | GH115 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 9671; End: 12178 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH115 | 34 | 811 | 4.5e-288 | 0.9956958393113343 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam15979 | Glyco_hydro_115 | 0.0 | 185 | 517 | 1 | 334 | Glycosyl hydrolase family 115. Glyco_hydro_115 is a family of glycoside hydrolases likely to have the activity of xylan a-1,2-glucuronidase, EC:3.2.1.131, or a-(4-O-methyl)-glucuronidase EC:3.2.1.-. |
| pfam17829 | GH115_C | 2.13e-61 | 664 | 827 | 1 | 172 | Gylcosyl hydrolase family 115 C-terminal domain. This domain is found at the C-terminus of glycosyl hydrolase family 115 proteins. This domain has a beta-sandwich fold. |
| pfam03648 | Glyco_hydro_67N | 7.30e-04 | 59 | 152 | 36 | 120 | Glycosyl hydrolase family 67 N-terminus. Alpha-glucuronidases, components of an ensemble of enzymes central to the recycling of photosynthetic biomass, remove the alpha-1,2 linked 4-O-methyl glucuronic acid from xylans. This family represents the N-terminal region of alpha-glucuronidase. The N-terminal domain forms a two-layer sandwich, each layer being formed by a beta sheet of five strands. A further two helices form part of the interface with the central, catalytic, module (pfam07488). |
| cd05399 | NT_Rel-Spo_like | 0.003 | 336 | 434 | 27 | 123 | Nucleotidyltransferase (NT) domain of RelA- and SpoT-like ppGpp synthetases and hydrolases. This family includes the catalytic domains of Escherichia coli ppGpp synthetase (RelA), ppGpp synthetase/hydrolase (SpoT), and related proteins. RelA synthesizes (p)ppGpp in response to amino-acid starvation and in association with ribosomes. (p)ppGpp triggers the bacterial stringent response. SpoT catalyzes (p)ppGpp synthesis under carbon limitation in a ribosome-independent manner. It also catalyzes (p)ppGpp degradation. Gram-negative bacteria have two enzymes involved in (p)ppGpp metabolism while most Gram-positive organisms have a single Rel-Spo enzyme (Rel), which both synthesizes and degrades (p)ppGpp. The Arabidopsis thaliana Rel-Spo proteins, At-RSH1,-2, and-3 appear to regulate a rapid (p)ppGpp-mediated response to pathogens and other stresses. This catalytic domain is found in association with an N-terminal HD domain and a C-terminal metal dependent phosphohydrolase domain (TGS). Some Rel-Spo proteins also have a C-terminal regulatory ACT domain. This subgroup belongs to the Pol beta-like NT superfamily. In the majority of enzymes in this superfamily, two carboxylates, Dx[D/E], together with a third more distal carboxylate, coordinate two divalent metal cations involved in a two-metal ion mechanism of nucleotide addition.Two of the three catalytic carboxylates are found in Rel-Spo enzymes, with the second carboxylate of the DXD motif missing. Evidence supports a single-cation synthetase mechanism. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| EDV05080.1 | 0.0 | 1 | 828 | 17 | 844 |
| ALJ61511.1 | 0.0 | 1 | 830 | 1 | 830 |
| QUT92919.1 | 0.0 | 1 | 830 | 1 | 830 |
| QDO71564.1 | 0.0 | 16 | 828 | 1 | 813 |
| QIU93956.1 | 0.0 | 13 | 828 | 10 | 820 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4C90_A | 1.06e-299 | 1 | 828 | 12 | 849 | Evidencethat GH115 alpha-glucuronidase activity is dependent on conformational flexibility [Bacteroides ovatus],4C90_B Evidence that GH115 alpha-glucuronidase activity is dependent on conformational flexibility [Bacteroides ovatus],4C91_A Evidence that GH115 alpha-glucuronidase activity is dependent on conformational flexibility [Bacteroides ovatus],4C91_B Evidence that GH115 alpha-glucuronidase activity is dependent on conformational flexibility [Bacteroides ovatus] |
| 7PUG_A | 7.96e-278 | 35 | 828 | 17 | 834 | ChainA, xylan alpha-1,2-glucuronidase [uncultured bacterium] |
| 7PXQ_A | 2.86e-275 | 35 | 835 | 16 | 840 | ChainA, xylan alpha-1,2-glucuronidase [uncultured bacterium] |
| 4ZMH_A | 4.77e-213 | 46 | 828 | 25 | 933 | Crystalstructure of a five-domain GH115 alpha-Glucuronidase from the Marine Bacterium Saccharophagus degradans 2-40T [Saccharophagus degradans 2-40],4ZMH_B Crystal structure of a five-domain GH115 alpha-Glucuronidase from the Marine Bacterium Saccharophagus degradans 2-40T [Saccharophagus degradans 2-40] |
| 6NPS_A | 4.40e-142 | 55 | 653 | 31 | 658 | Crystalstructure of GH115 enzyme AxyAgu115A from Amphibacillus xylanus [Amphibacillus xylanus NBRC 15112],6NPS_B Crystal structure of GH115 enzyme AxyAgu115A from Amphibacillus xylanus [Amphibacillus xylanus NBRC 15112] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
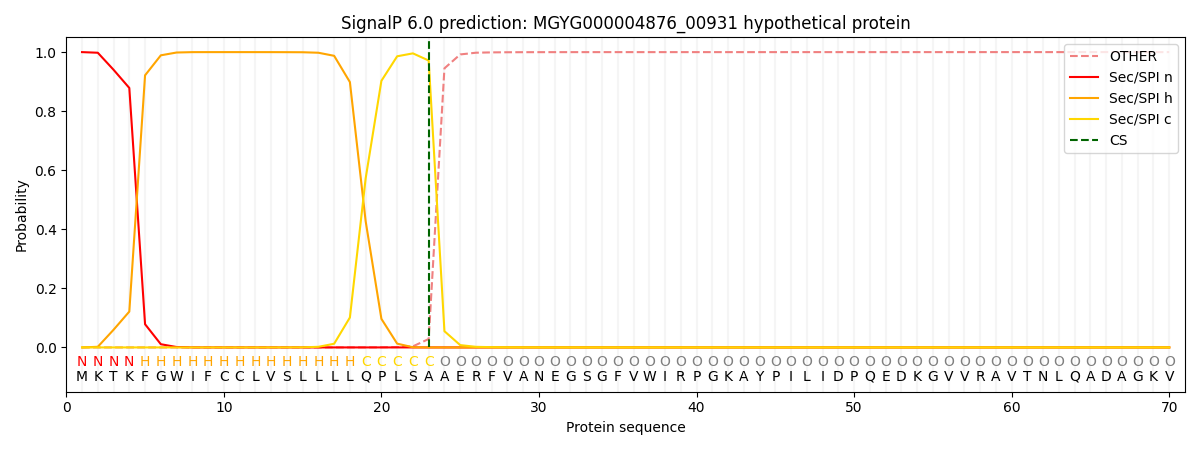
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000430 | 0.998873 | 0.000227 | 0.000148 | 0.000142 | 0.000152 |
