You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004892_00283
You are here: Home > Sequence: MGYG000004892_00283
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
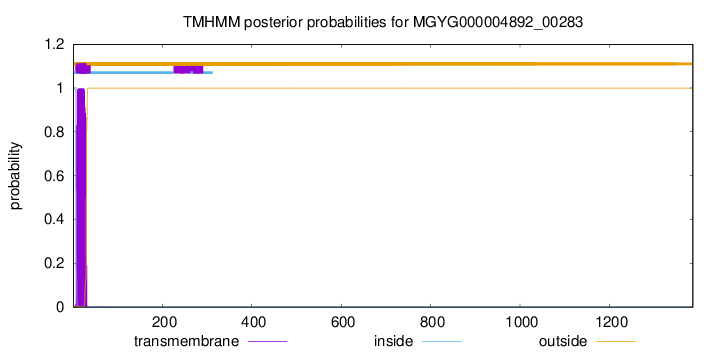
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Acutalibacteraceae; UMGS1858; | |||||||||||
| CAZyme ID | MGYG000004892_00283 | |||||||||||
| CAZy Family | CE2 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 334; End: 4497 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE2 | 804 | 1010 | 1.8e-44 | 0.9952153110047847 |
| CE2 | 161 | 367 | 4.6e-42 | 0.9952153110047847 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd01831 | Endoglucanase_E_like | 3.29e-28 | 161 | 367 | 1 | 168 | Endoglucanase E-like members of the SGNH hydrolase family; Endoglucanase E catalyzes the endohydrolysis of 1,4-beta-glucosidic linkages in cellulose, lichenin and cereal beta-D-glucans. |
| cd01831 | Endoglucanase_E_like | 3.29e-27 | 804 | 1010 | 1 | 168 | Endoglucanase E-like members of the SGNH hydrolase family; Endoglucanase E catalyzes the endohydrolysis of 1,4-beta-glucosidic linkages in cellulose, lichenin and cereal beta-D-glucans. |
| pfam17996 | CE2_N | 2.33e-20 | 43 | 152 | 3 | 106 | Carbohydrate esterase 2 N-terminal. This is the N-terminal beta-sheet domain with jelly roll topology found in CE2 acetyl-esterase from the bacterium Clostridium thermocellum. This enzyme displays dual activities, it catalyses the deacetylation of plant polysaccharides and also potentiates the activity of its appended cellulase catalytic module through its noncatalytic cellulose binding function. This N-terminal jelly-roll domain appears to extend the substrate/cellulose binding cleft of the catalytic domain in C.thermocellum. |
| pfam17996 | CE2_N | 6.36e-17 | 686 | 795 | 3 | 106 | Carbohydrate esterase 2 N-terminal. This is the N-terminal beta-sheet domain with jelly roll topology found in CE2 acetyl-esterase from the bacterium Clostridium thermocellum. This enzyme displays dual activities, it catalyses the deacetylation of plant polysaccharides and also potentiates the activity of its appended cellulase catalytic module through its noncatalytic cellulose binding function. This N-terminal jelly-roll domain appears to extend the substrate/cellulose binding cleft of the catalytic domain in C.thermocellum. |
| cd14256 | Dockerin_I | 1.39e-12 | 1317 | 1371 | 1 | 54 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBL33310.1 | 4.28e-35 | 680 | 1016 | 12 | 357 |
| CQR54111.1 | 3.41e-34 | 686 | 1016 | 19 | 356 |
| CBK96966.1 | 6.53e-34 | 680 | 1016 | 12 | 357 |
| AIQ28226.1 | 1.15e-33 | 681 | 1016 | 16 | 356 |
| AIQ38796.1 | 1.09e-31 | 686 | 1016 | 19 | 356 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2WAO_A | 4.51e-23 | 699 | 1016 | 29 | 333 | ChainA, ENDOGLUCANASE E [Acetivibrio thermocellus] |
| 2WAB_A | 1.10e-22 | 699 | 1016 | 29 | 333 | ChainA, ENDOGLUCANASE E [Acetivibrio thermocellus] |
| 3U37_A | 1.75e-20 | 803 | 1016 | 166 | 406 | AnAcetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],3U37_B An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],3U37_C An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],3U37_D An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],3U37_E An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],3U37_F An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],3U37_G An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],3U37_H An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316] |
| 4DEV_A | 3.22e-19 | 803 | 1016 | 166 | 406 | AnAcetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],4DEV_B An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],4DEV_C An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],4DEV_D An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],4DEV_E An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],4DEV_F An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],4DEV_G An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316],4DEV_H An Acetyl Xylan Esterase (Est2A) from the Rumen Bacterium Butyrivibrio proteoclasticus. [Butyrivibrio proteoclasticus B316] |
| 4XVH_A | 7.42e-18 | 690 | 1016 | 8 | 329 | Crystalstructure of a Corynascus thermopiles (Myceliophthora fergusii) carbohydrate esterase family 2 (CE2) enzyme plus carbohydrate binding domain (CBD) [Chaetomium] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P10477 | 5.49e-21 | 699 | 1016 | 510 | 814 | Cellulase/esterase CelE OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=celE PE=1 SV=2 |
| B3PDE5 | 1.58e-09 | 744 | 1015 | 87 | 358 | Acetylxylan esterase / glucomannan deacetylase OS=Cellvibrio japonicus (strain Ueda107) OX=498211 GN=ce2C PE=1 SV=1 |
| A0A3R0A696 | 1.30e-06 | 492 | 670 | 819 | 999 | Alpha-L-arabinofuranosidase OS=Bifidobacterium longum subsp. longum (strain ATCC 15707 / DSM 20219 / JCM 1217 / NCTC 11818 / E194b) OX=565042 GN=blArafA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
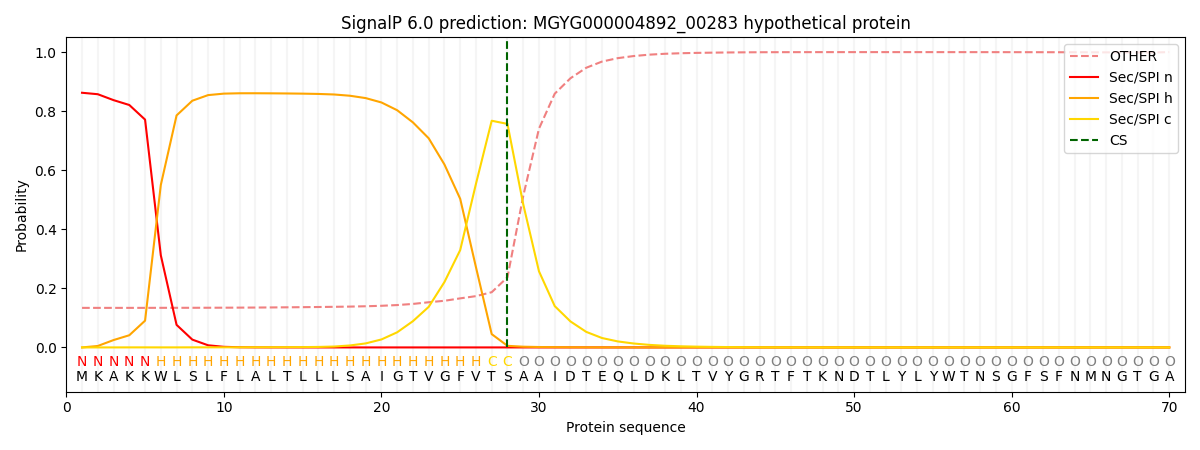
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.141314 | 0.852531 | 0.004789 | 0.000551 | 0.000370 | 0.000426 |