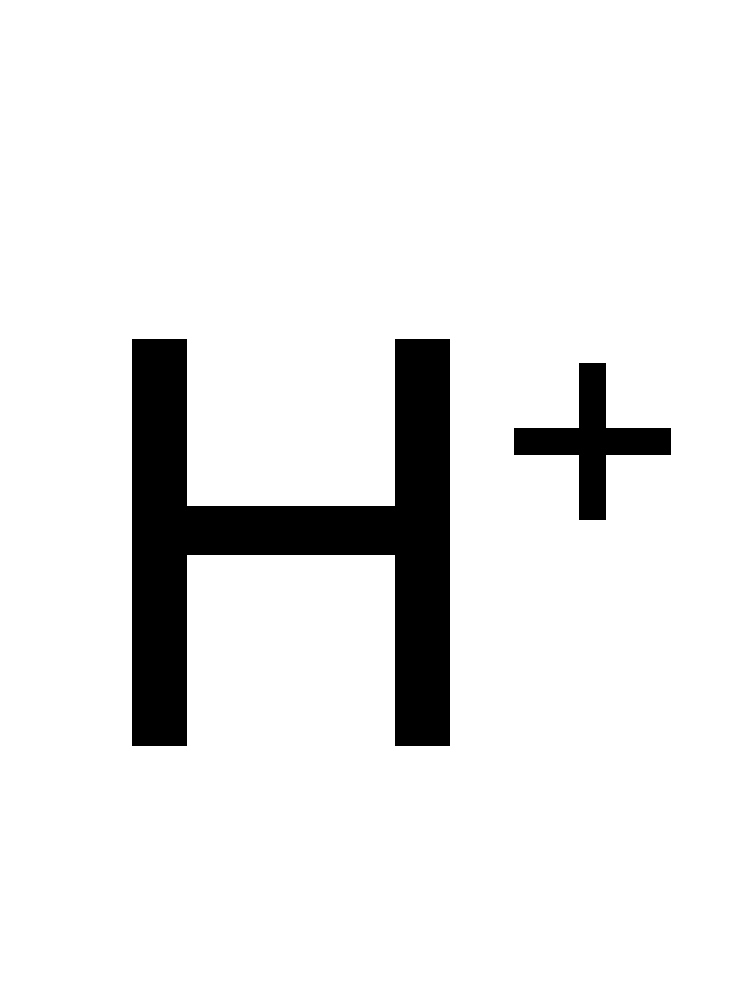| Title | Kinetic and Spectroscopic Studies on the Quercetin 2,3-Dioxygenase from Bacillus subtilis |
|---|---|
| Author | Matthew R. Schaab, Brett M. Barney, and Wilson A. Francisco |
| DOI | 10.1021/bi051571c |
| Abstract | Quercetin 2,3-dioxygenase from Bacillus subtilis (QueD) converts the flavonol quercetin and molecular oxygen to 2-protocatechuoylphloroglucinolcarboxylic acid and carbon monoxide. QueD, the only known quercetin 2,3-dioxygenase from a prokaryotic organism, has been described as an Fe2+-dependent bicupin dioxygenase. Metal-substituted QueDs were generated by expressing the enzyme in Escherichia coli grown on minimal media in the presence of a number of divalent metals. The addition of Mn2+, Co2+, and Cu2+ generated active enzymes, but the addition of Zn2+, Fe2+, and Cd2+ did not increase quercetinase activity to any significant level over a control in which no divalent ions were added to the media. The Mn2+- and Co2+-containing QueDs were purified, characterized by metal analysis and EPR spectroscopy, and studied by steady-state kinetics. Mn2+ was found to be incorporated nearly stoichiometrically to the two cupin motifs. The hyperfine coupling constant of the g = 2 signal in the EPR spectra of the Mn2+-containing enzyme showed that the two Mn2+ ions are ligated in an octahedral coordination. The turnover number of this enzyme was found to be in the order of 25 s-1, nearly 40-fold higher than that of the Fe2+-containing enzyme and similar in magnitude to that of the Cu2+-containing quercertin 2,3-dioxygenase from Aspergillus japonicus. In addition, kinetic and spectroscopic data suggest that the catalytic mechanism of QueD is different from that of the Aspergillus quercetinases but similar to that proposed for the extradiol catechol dioxygenases. This study provides evidence that Mn2+ might be the preferred cofactor for this enzyme and identifies QueD as a new member of the manganese dioxygenase family. |
Uniprot ID: P42106
Protein: Quercetin 2,3-dioxygenase
Organism: Bacillus subtilis (strain 168)
Length: 337 AA
Taxonomic identifier: 224308 [NCBI]
| Source | Domain | Start | End | E-value (Domain) | Coverage |
|---|---|---|---|---|---|
| Pfam-A | Cupin_2 | 55 | 110 | 7.1e-13 | 0.775 |
| Pfam-A | Cupin_2 | 226 | 281 | 5.1e-16 | 0.775 |
Program: hmmscan
Version: 3.1b2 (February 2015)
Method: hmmscan --domtblout hmmscan.tbl --noali -E 1e-5 pfam query.fa
Date: Mon Jul 20 14:32:16 2020
Description:
Cupin_2
This family represents the conserved barrel domain of the ‘cupin’ superfamily1 (‘cupa’ is the Latin term for a small barrel).
This family represents the conserved barrel domain of the cupin superfamily1 (cupa is the Latin term for a small barrel).
Information is taken from Pfam and InterPro web site.
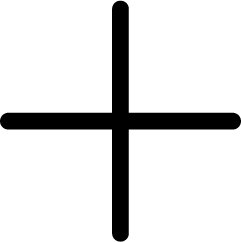
quercetin + oxygen ⇒ 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate + carbon monoxide + H+
![[L1]quercetin](../static/images/chemical_structure/OR8/OR8_1/[L1]quercetin.png)
![[L2]oxygen](../static/images/chemical_structure/OR8/OR8_1/[L2]oxygen.png)

![[R1]2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate](../static/images/chemical_structure/OR8/OR8_1/[R1]2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate.png)

![[R2]carbon monoxide](../static/images/chemical_structure/OR8/OR8_1/[R2]carbon monoxide.png)